Deeplex® Myc-TB: Cost-saving & cost-effective TB resistance detection
A recent study conducted in South Africa demonstrates the clinical value and cost-effectiveness of targeted next-generation sequencing for tuberculosis drug resistance testing, using Deeplex Myc-TB. It demonstrates that this approach improves the speed and accuracy of diagnosis, optimizes treatment management, reduces infectiousness and delivers measurable benefits for public health and healthcare costs.
Drug-resistant tuberculosis: an urgent challenge for global health
Tuberculosis remains one of the world’s deadliest infectious diseases, particularly affecting countries with high incidence rates such as South Africa. Drug resistance is a major obstacle to treatment, especially in patients with multidrug-resistant tuberculosis (MDR-TB) or extensively drug-resistant tuberculosis (XDR-TB).
Current diagnostic algorithms combining cartridge-based molecular tests and phenotypic drug susceptibility testing (pDST) have significant limitations. These molecular tests target only some common resistance mutations and do not cover the newest anti-tuberculosis drugs, for which pDST faces long turnaround times. As a result, diagnostic delays are prolonged, frequently leading to inappropriate initial treatments, poorer patient outcomes and extended periods of infectiousness.
Targeted next-generation sequencing (tNGS) offers the potential for a paradigmatic shift in tuberculosis DST. It enables detection of resistance to almost all anti-tuberculosis drugs in a single assay with a short turnaround time.
On this basis, this study evaluated the potential of tNGS as a more effective approach for rapid and accurate identification of drug resistance in Mycobacterium tuberculosis in South Africa. Deeplex Myc-TB testing on an Illumina sequencing platform was utilized as the tNGS model, in accordance with guidance from the National Institute for Communicable Diseases in this country.
Approach to assess cost-effectiveness
The authors conducted a modeled cost-effectiveness analysis comparing Deeplex Myc-TB-based tNGS with the current standard of care for DST of rifampicin resistant tuberculosis, including Xpert MTB/XDR for detection of resistance to isoniazid and fluoroquinolones and pDST for bedaquiline and linezolid. The analysis incorporated diagnostic performance, time to appropriate treatment initiation, clinical outcomes (including cure, failure, or death), secondary transmission, and direct healthcare costs. Population-level effects were quantified in disability-adjusted life years (DALYs), based on a projected annual cohort of 10 000 patients with rifampicin-resistant TB. Several scenarios were evaluated, including centralized and decentralized tNGS implementation models, to provide realistic estimates of the potential public health and economic impact of tNGS.
Measurable clinical and economic benefits of tNGS for TB drug resistance testing
The modeling results show that tNGS provides clear clinical and economic benefits for tuberculosis drug resistance testing compared with the current standard of care.
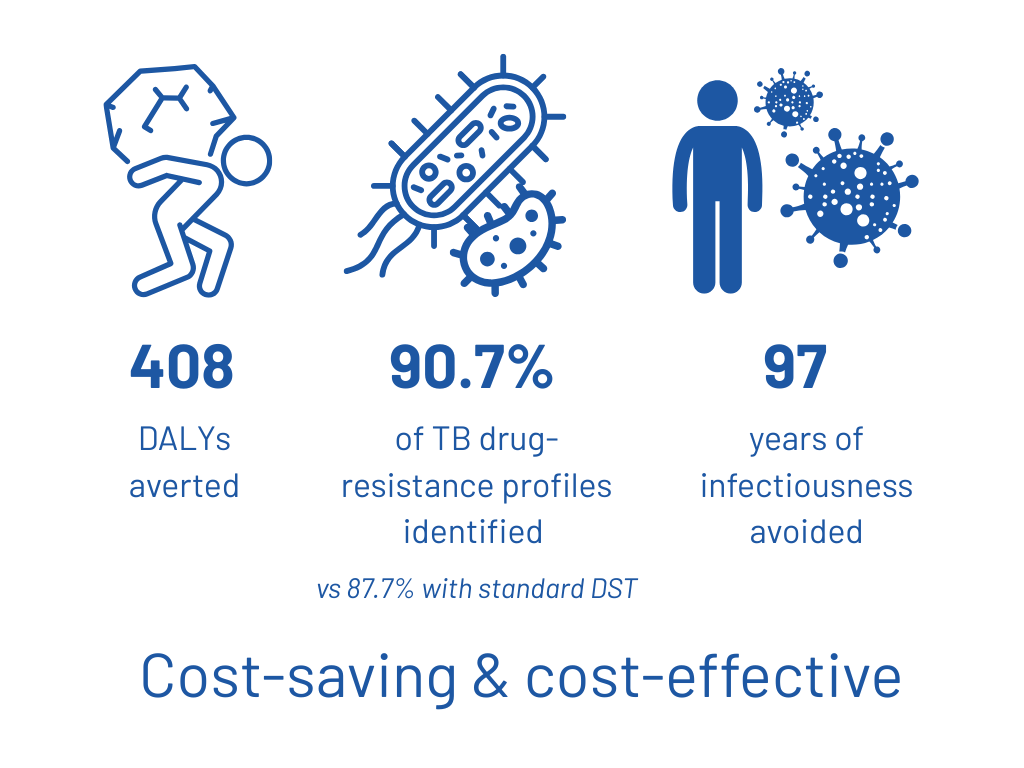
According to the model, tNGS correctly identified 90.7% of resistance profiles, compared with 87.7% for the standard of care, thereby limiting treatment failures linked to undetected resistance.
As a result, the use of tNGS prevented a total of 408 DALYs, corresponding to 408 years of healthy life gained, due to reductions in mortality and complications associated with inappropriate treatment.
In addition, earlier and more accurate resistance detection averted 97 years of infectious time, thereby reducing tuberculosis transmission at the population level.
These individual and public health benefits were accompanied by measurable economic gains, with tNGS shown to be substantially cost-saving in centralized scenarios and cost-effective in decentralized settings
Deeplex® Myc-TB : The optimal tNGS solution for rapid TB drug resistance testing
Deeplex Myc-TB is the pioneering tNGS-based solution for tuberculosis drug resistance testing. The test relies on targeted deep sequencing combined with automated data analysis and interpretation through the secure Deeplex Myc-TB web application. This fully integrated, culture-free diagnostic kit delivers molecular results on resistance or susceptibility to 15 anti-tuberculosis drugs in a single test, with a turnaround time of less than 48 hours.
Deeplex Myc-TB has been endorsed by the World Health Organization (WHO) as the only targeted NGS (tNGS) assay that meets performance criteria for all 10 drugs assessed in an independent evaluation. As such, it is the only tNGS test recommended by the WHO for detecting resistance in both drug-susceptible and multidrug-resistant/rifampicin-resistant tuberculosis.
By enabling rapid and comprehensive detection of genetic resistance to anti-tuberculosis drugs, this innovative assay serves as a powerful tool to guide timely and effective clinical decision-making for patients with tuberculosis. As shown by the above study, its use contributes to improved treatment outcomes, reduced infectious periods, limited tuberculosis transmission, and optimized use of healthcare resources.
Source


